1) Which element is oxidized
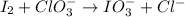
Oxidation numbers
I2: 0
ClO3-: for Cl +5 and for O is -2
IO3-: for I is +5 and for O -2
Cl-: -1.
According to the oxidation number
a. Iodine (I) has been oxidized. It changed from 0 to +5.
b. Chlorine has been reduced. It changed from +5 to -1
2) reducing agent and oxidizing agent
c. A reducing agent donates electrons. In the reaction, Iodine is the reducing agent.
d. An oxidizing agent accepts electrons. In the reaction, Chlñorine is the oxidizing agent.
3) Balancing the chemical equation
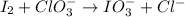
Step 1: break the reaction into two half-reactions.
Oxidation half-reaction
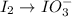
Reduction half-reaction
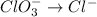
Step 2: balance all elements EXCEPT for hydrogen and oxygen
Oxidation half-reaction
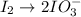
Reduction half-reaction
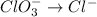
Step 3: Balance OXYGEN. We do so by adding water molecules to the half-reactions as needed.
Oxidation half-reaction
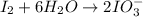
Reduction half-reaction
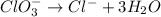
Step 4: Balance HYDROGEN. We do so by adding protons (H+) to the half-reactions as needed.
Oxidation half-reaction
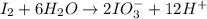
Reduction half-reaction
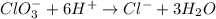
Step 5: Balance CHARGES. We do so by adding electrons
Oxidation half-reaction
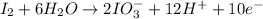
Reduction half-reaction
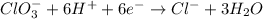
Step 6: Multiply each half-reaction in such a way we can cancel the electrons.
Oxidation half-reaction
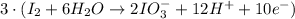
New oxidation half-reaction
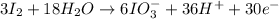
Reduction half-reaction
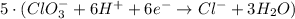
New reduction half-reaction
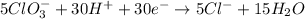
Step 7: combine the half-reactions. We have 30e in the reactants and 30e in the products. We can cancel them and combine the remaining species.
New oxidation half-reaction
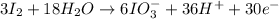
New reduction half-reaction
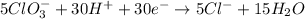
Overall reaction
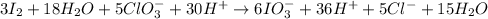
Step 8: Balance the reaction by reducing the number of water molecules and protons.
Overall reaction
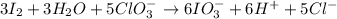
e. Balance the reaction
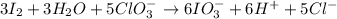
.