ANSWER
Products, reactants
Step-by-step explanation
An endothermic reaction is defined as a type of reaction in which heat is absorbed from the surroundings. In an endothermic reaction, the change in enthalpy is always positive.
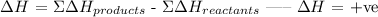
Therefore, in an endothermic reaction, the products have more energy than the reactants.
Hence, the correct answer is option C