Answer: the mass of S contained in 1.46 x 10^24 atoms of this element is 77.6g. The best option to answer the question is letter A.
Step-by-step explanation:
The question requires us to determine the mass of sulfur contained in 1.46 × 10^24 atoms of this element.
To solve this problem, we can apply the Avogadro's number: it defines the number of particles (ions, atoms, molecules etc.) in one mol of any substance. Then, we'll need to use the atomic mass of sulfur (S) to determine the mass of the number of moles calculated.
According to the Avogadro's number, there are 6.022 x 10^23 atoms of S in 1 mol of this atom. Thus, we can write:
6.022 x 10^23 atoms ------------------------- 1 mol S
1.46 x 10^24 atoms --------------------------- x
Solving for x, we'll have:
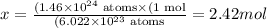
Therefore, there are 2.42 moles of S in 1.46 x 10^24 atoms of this element.
Now, knowing that the atomic mass of S is 32.065 amu (molar mass = 32.065 g/mol), we can use the following equation to calculate the required mass:
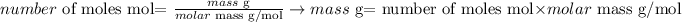
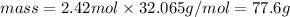
Therefore, the mass of S contained in 1.46 x 10^24 atoms of this element is 77.6g. The best option to answer the question is letter a.