Answer:
The volume of the bubble when it reaches the surface is 1,186.2mL.
Step-by-step explanation:
The data of the initial state of the bubble is:
• V1= 250mL
,
• P1= 2.42atm
,
• T1= 15°C
The data of the final state of the bubble is:
• V2= this is what we are going to calculate.
,
• P2= 0.95atm
,
• T2=28°C
Now, with the ideal gas formula we can calculate the final volume of the bubble:
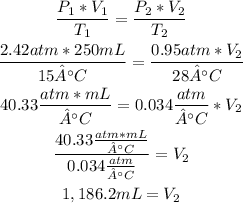
So, the volume of the bubble when it reaches the surface is 1,186.2mL.