- Kc for the a) reaction:
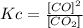
- Kc for the b) reaction:
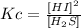
- Kc for the c) reaction:
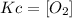
To write the expression for the equilibrium constant (kc), it is important to remember that:
- The concentration of the products is multiplied in the numerator, while the concentration of the reactants is multiplied in the denominator.
- Only the concentrations of the gaseous and/or dissolved species should be included.