The first step to solve this question is to convert the given mass of nitrogen to moles:
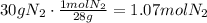
Use the stoichiometric ratio to find how many moles of NH3 are produced:
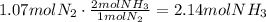
Use the ideal gases law to find the volume of ammonia produced:
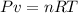
Solve the equation for v and replace for the known values (since the gas is at standard temperature and pressure, T is 273K and P is 1atm). Remember that R is the ideal gas constant and has a value of 0.082atmL/molK:
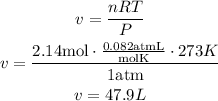
47.9L of ammonia are produced.