Answer:
6.37.
Step-by-step explanation:
What is given?
[H (+)] = 4.25 x 10⁻⁷ mol/dm³
Step-by-step solution:
First, let's see the formula to calculate the pH based on the hydrogen ion concentration (H (+)):
![pH=-log\lbrack H^+].](https://img.qammunity.org/2023/formulas/chemistry/college/p7evooktvpvfrgxih0hw9czsl462qa1utf.png)
*Logarithm must be in base 10!
Our given concentration of hydrogen ion is 4.25 x 10⁻⁷ mol/dm³. Remember that the units of concentration must be in M (mol/L) but in this case, 1 dm³ equals 1 L, so based on this, we have that:
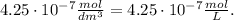
So, we can replace this value in the formula of pH:
![pH=-log\lbrack4.25\cdot10^(-7)]\approx6.37.](https://img.qammunity.org/2023/formulas/chemistry/college/svfrvip44k58ksz2002hvbwdtxpl5ptye1.png)
The pH of milk would be 6.37 which means that is slightly acid (weak acid).