Answer:
9.24 g of KCl.
Step-by-step explanation:
What is given?
Mass of Fe(NO3)3 = 10.0 g.
Molar mass of Fe(NO3)3 = 242 g/mol.
Mlar mass of KcCl = 74.5 g/mol. (you can calculate the molar massesof a compound using the periodc table)
Step-by-step solution:
Let's covert 110.0 g of Fe(NO3)3 to moles using is molar mass, like this:
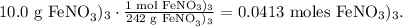
You can see in the chemical equation that 3 moles of KCl are required to react with 1 mol of Fe(NO3)3, so by doing a rule o three to find how many moles of KClowe require to react with 0.0413 moles Fe(NO3)3, the calculation will look this:
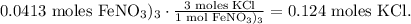
And the final step is to convert 0.124 moles of KCl to grams using its given molar mass, likethis:
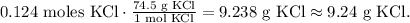
We will require 9.24 g of KCl to react with 10.0 g of Fe(NO3)3.