We begin by balancing the equation by trial and error. We will start by balancing the hydrogens. We have 2 hydrogens on the reactants side, so we will write the coefficient 2 before HNO2:
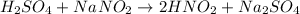
Now we will continue with the sodium. On the product side, we have two sodium atoms. To balance it we put the coefficient 2 before NaNO2:
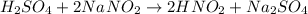
Let's count how many atoms we have of each element:
We have the same number of atoms of each element on each side of the reaction, so the equation is balanced. Now the draw of the molecules is: