ANSWER
Step-by-step explanation
Given information
The thermal energy added is 3000 J
The mass of the metal block is 250.0 grams
The temperature change is 37.5 degrees Celcius
Firstly, we need to find the specific heat capacity of the metal block
To find the specific heat capacity of the metal block, follow the steps below
Step 1: Write the formula for calculating the heat of a substance
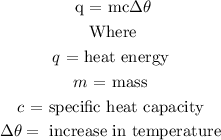
Step 2: Substitute the given data into the formula in step 1 to find c
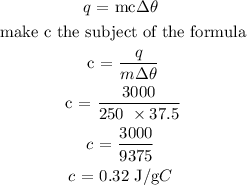
Hence, the specific heat capacity of the metal block is 0.32 J/g degrees Celcius
According to the table of specific heats, the metal that has the above specific heats is Germanium