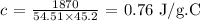Answer:
Step-by-step explanation:
Here, we want to calculate the specific heat capacity of the metal
Mathematically:
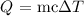
where :
Q is the amount of heat absorbed given as 1870 J
m is the mass of the metal given as 54.51g
c is the specific heat capacity that we want to calculate
delta T is the temperature change given as 45.2 °C
Rewriting the formula above in terms of c, we have it that:
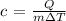
Substituting the values, we have it that: