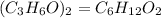Answer:
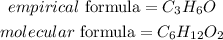
Explanations:
Given the following parameters
Mass of ethyl butanoate = 2.78grams
Mass of CO2 = 6.32grams
Mass of H2O = 2.58grams
Determine the mole of CO2 and H2O
Mole of CO2 = mass/molar mass
Mole of CO2 = 6.32/44.01
Mole of CO2 = 0.144moles
Since there are 1 mole of carbon in CO2, the mass of carbon present will be:
Mass of C = mole * molar mass
Mass of C = 0.144 * 12
Mass of C = 1.728grams
Mole of H2O = mass/molar mass
Mole of H2O = 2.58/18
Mole of H2O = 0.143moles
Since there are 2 atoms of hydrogen in H2O, the required mass of hydrogen will be:
Mass of H = 2(0.143) * 1
Mass of H = 0.287grams
Determine the mass of oxygen
Mass of oxygen =Total mass - (grams of C + grams of H)
Mass of oxygen = 2.78 - (1.728 +0.287)
Mass of oxygen = 0.765grams
Convert the number of grams to moles
Mole of Carbon = mass/molar mass
mole of carbon = 1.728/12 = 0.143moles
Mole of Hydrogen = 0.287/1 = 0.287moles
Mole of Oxygen = 0.77/16 = 0.04813moles
Divide the number of moles of each element by the smallest number obtained
Ratio of Carbon = 0.143/0.04813 = 2.97 ≈ 3
Ratio of Hydrogen = 0.287/0.04813 = 5.963 ≈ 6
Ratio of Oxygen = 0.04813/0.04813 = 1
Hence the empirical formula for the compound is C₃H₆O
Determine the molecular mass of the compound
![\begin{gathered} (C_3H_6O)_n=116 \\ [3(12)+1(6)+16]n=116 \\ (36+6+16)n=116 \\ 58n=116 \\ n=(116)/(58) \\ n=2 \end{gathered}](https://img.qammunity.org/2023/formulas/chemistry/college/4iekpuqf7p0p0z527ydawf9vsrz3xi4tpx.png)
The molecular formula will be expressed as: