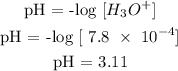Category --------- General chemistry
Sub-category ------- Acid and Base pH
ANSWER
Solution A ------- 9.35
Solution B ---------- 7.1 x 10^-7 mol/L
solution C ---------- 3.11
Step-by-step explanation
pH measures the degree of acidity and alkalinity of a substance.
For part A
The hydroxonium ion of the solution is 4.5 x 10^-10 mol/L
Recall,
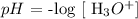
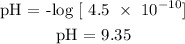
For solution B
The pH of the solution is 6.15
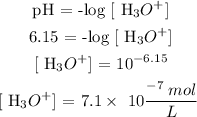
For solution C
The hydroxonium ion is 7.8 x 10^-4 mol/L