Answer:
34.68g of Lithium are needed.
Step-by-step explanation:
1st) It is necessary to write the balanced chemical equation:
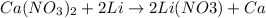
From the stoichiometry of the reaction we know that 1 mole of Calcium nitrate reacts with 2 moles of lithium.
2nd) It is necessary to use the molar mass of Ca(NO3)2 (164.09g/mol) and Li (6.94g/mol) to convert moles to grams:
• Ca(NO3)2 conversion: It is 1 mole, so the conversion is 164.09g,.
• Li conversion,:
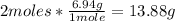
Now we know that 164.09g of Ca(NO3)2 react with 13.88g of Li.
3rd) Using a mathematical rule of three and with the grams of Ca(NO3)2 and Li, we can calculate the amount of lithium needed:
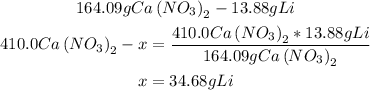
Finally, 34.68g of Lithium are needed.