The first step to solve this question is to convert the given masses to moles using the corresponding molecular weight:
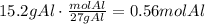
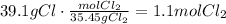
Divide each of the results by the coefficient of each reactant in the equation. It means, divide the amount of Al by 2 and the amount of Cl2 by 3:
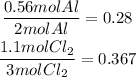
It means that the limiting reactant is Aluminium and we have to base our calculations on this reactant. From the equation we know than 2 moles of Al produce 2 moles of AlCl3.
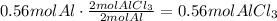
Now, convert this amount of AlCl3 to mass using its molecular weight:
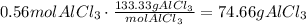
It means that the mass of AlCl3 formed is 74.66g.