Given:
The mass of ice is m = 50 g
The initial temperature of ice is
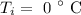
The final temperature of the water is
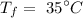
Step-by-step explanation:
Heat is first required to convert ice to water at 0 degrees Celsius.
The heat required to convert ice to water at 0 degrees Celsius can be calculated by the formula
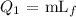
Here, the latent heat of fusion is
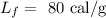
On substituting the values, the heat will be
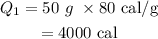
Now, the heat required to increase the temperature of water from 0 degrees Celsius to 35 degrees Celsius can be calculated by the formula
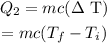
Here, c is the specific heat of water at 1 cal/g deg C
On substituting the values, the heat required to increase the temperature will be
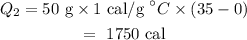
The total heat will be
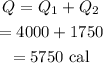
Final Answer:
The heat required to convert 50 grams of ice to water from 0 degrees Celsius to 35 degrees Celsius is 5750 cal