A combustion reaction consists of oxidizing a fuel using oxygen as the oxidizing agent. As products, we will have CO2 and water. Therefore a combustion reaction must have the following components:
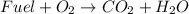
Now, we will compare each option that we are given to determine if they are a combustion reaction or not.
1. The presence of oxygen is not seen in the reaction, there is also no formation of CO2, so this is not a combustion reaction.
2. This is a particular case. We have a fuel that in this case is hydrogen that reacts with oxygen to form water. This is not such a combustion reaction since no CO2 is produced.
3. This is the same reaction as above, only the moles are divided by two, it is not considered a combustion reaction either.
4. In this reaction, the states of the reactants are incorrect, it already tells us that we have liquid H2 and liquid oxygen. Bringing these gases to these conditions is very difficult and requires quite a high energy expenditure.
5. This is a combustion reaction, we have methane as fuel, oxygen and CO2, and water as products.
So the answer will be: 5