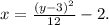From the graph, we notice that the parabola has to be a horizontal parabola with vertex at:
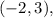
that opens to the right.
Recall that the standard form of a horizontal parabola is:
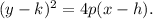
Where, (h,k) are the coordinates of the vertex, and p is the distance to the vertex to focus.
Substituting the vertex in the above equation, we get:
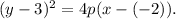
From the diagram, we get that:
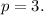
Therefore:
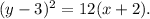
Solving the above equation for x, we get:
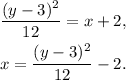
Answer: