Answer:
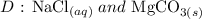
Step-by-step explanation:
Here, we want to get the product from the double displacement reaction
A double displacement reaction also known as a double decomposition reaction involves the exchange of anions between the metallic elements to produce a solid precipitate and an aqueous solution alongside
We have the equation of reaction as follows:
