Given the equation system:
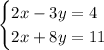
To determine if the coordinate pair (5,2) is a solution of the equation system you have to replace each equation with the x-coordinate and calculate them for y.
If the obtained values of y are equal to the y-coordinate, then the given coordinate pair is a solution for the equation system.
Another method is to solve the equation system using, for example, the substitution method and comparing your results with the given coordinate pair.
I'm going to solve the equation system using the substitution method.
1) Solve the first equation for x:
-Pass the y-term to the right side of the equal sign:
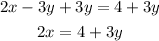
-Divide both sides by 2
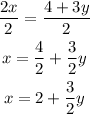
2) Replace the expression obtained for x in the second expression and solve for y:
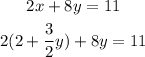
-Distribute the multiplication on the parentheses term, which means that you have to multiply both terms by 2
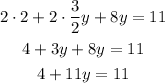
-Pass 4 to the right side of the equation by applying the opposite operation
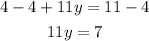
-Divide both sides by 5
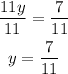
3)Now that you have determined the value of y, you can calculate the value of x by replacing y=7/11 in the expression obtained for x in the first step
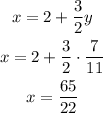
The solution of the system is the coordinate pair
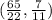
The given coordinate pair is NOT a solution of the equation system.