Answer
3.25 x 10²⁴ molecules
Step-by-step explanation
Given:
Mass of H₂O = 97.2 g
Atomic mass: (H = 1.008 g/mol, O = 16.00 g/mol)
Avogadro's Number = 6.02 x 10²³ molecules/mole
What to find:
The number of molecules of H₂O equivalent to 97.2 g H₂O.
Step-by-step solution:
The first step is to determine the number of moles of H₂O in 97.2 g H₂O.
Let x represents the number of moles in 97.2 g H₂O
1 mole of water is equivalent to (2 x 1.008 g + 16.00 g) = 18.016 g of H₂O
So x mole of water is equivalent to 97.2 g of H₂O
Cross multiply
(1 mole x 97.2 g) = x mole x 18.016 g
Divide both sides by 18.016 g
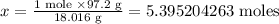
Hence, 5.395204263 moles of H₂O are equivalent to 97.2 g of H₂O.
The final step is to determine the number of molecules in 5.395204263 moles of H₂O.
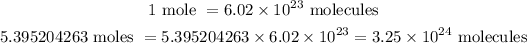
Therefore, there