Answer:
1. (unpaired electrons)
Step-by-step explanation:
Fluorine (F) has 9 protons (atomic number), so as this element is neutral, it has 9 electrons, so let's write its electronic configuration:
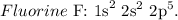
Now, let's draw the electron distribution of F:
You can realize that we just have one unpaired electron in the fluorine atom, so the answer would be 1. (unpaired electrons)