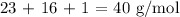Answer:
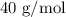
Step-by-step explanation:
Here, we want to get the gram formula mass of one mole of sodium hydroxide
The gram formula mass is equivalent to the molar mass of sodium hydroxide
To get the molar mass of sodium hydroxide, we need to add the atomic masses of the individual elements
Mathematically, we have that as: