Answer:
1548.5cubic centimeters.
Explanations:
According to Charles law, the volume of a given mass is directly proportional to the temperature provided that the pressure is constant. Mathematically;
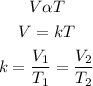
where
V1 and V2 are the volumes
T1 and T2 are the temperatures
Given the following parameters
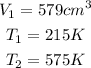
Required
New volume V2
Substitute
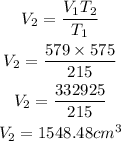
Hence the new volume rounded to nearest one decimal place is 1548.5cubic centimeters.