Step 1 - Understanding the relation between pressure, volume and temperature for a gas
Three variables are very important for determining the state of a gas: temperature (T), pressure (p) and volume (V). They are all related by the ideal gas equation:
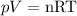
In this equation, n represents the number of moles of gas and R is the universal gas constant.
Step 2 - Understanding what is happening inside the balloon
Inside the balloon, the number of moles of gas will not vary. Therefore, we can rewrite the ideal gas equation as:
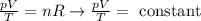
We know that the balloon is being cooled, therefore T is decreased. At the same time, the volume is increasing. Let's think of the equation this way:
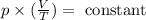
Since T is decreasing and V is increasing the term in parenthesis is also increasing (a lot). But because the multiplication of these two terms must be always the same (constant), the term in parenthesis must be decreased by the pressure.
Otherwise, it would increase indefinitely, and the result wouldn't be constant. Therefore, the only possible thing that can happen to the pressure is decreasing. The correct alternative is thus c) it decreases.