Answer
176.16 kJ
Step-by-step explanation
Given:
Mass of NH3 = 10 grams
Equation: 4NH3 + 5O2-> 4NO + 6H2O ΔH = 1, 200 kJ
What to find:
The heat released when 10 grams of NH3 reacts with excess O2.
Step-by-step solution:
The first step is to convert the 10 grams of NH3 that reacts to mole using the mole formula
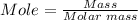
Molar mass of NH3 = 17.031 g/mol
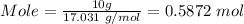
Now, you can use the mole of NH3 and the given equation to calculate the heat released.
From the equation;
4 moles of NH3 released 1,200 kJ of heat
Therefore, 0.5872 moles of NH3 will release
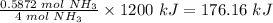
Therefore, the heat released when 10 grams of NH3 reacts with excess O2 is 176.16 kJ