Given,
The work function of the metal, W=4.48 eV
The work function is given by the formula,
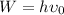
Where ν₀ is the threshold frequency of the metal.
But the frequency is related to wavelength as,
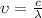
Where c is the speed of light and λ is the wavelength.
Thus the work function will be,
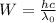
Where λ₀ is the maximum wavelength needed to eject the electron from the given metal.
On substituting the known values in the above equation,

Thus the maximum wavelength of the light required is 277.48 nm.
The frequency of the light is,
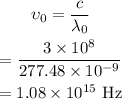
Thus the frequency of the required light is 1.08×10¹⁵ Hz.