ANSWER:
Paralell: -4
Perpendicular: 1/4
Explanation:
We have the following equation:

Now, we have that an equation of a line in its slope-intercept form has the following form:
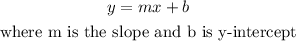
We apply it in this case:

When two lines are parallel, the slope is the same, while when they are perpendicular, the product of the slopes is equal to -1, we calculate each case below as follows:
