Given:
The current drawn by the heating element is: I = 18.014 A.
Mass of the water is: m = 176.251 g.
The temperature difference of water is: T = 87.714 deg C - 20 deg C = 67.714 deg C.
Time for which the change in temperature occurs: t = 7.167 minutes
The specific heat of water is: c = 4.186 J/g degC.
To find:
The resistance of the heating element.
Step-by-step explanation:
The expression for the heat is given as:
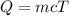
Substitute the values in the above equation, we get:
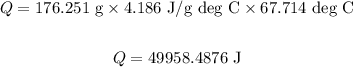
Joule's equation for electric heating is given as:
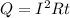
Here, R is the resistance of the heating element.
Rearranging the above equation, we get:
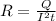
Substituting the values in the above equation, we get:

Final Answer:
The resistance of the heating element is 0.3580 Ω.