Chemistry => Chemical Reactions => Balancing equations
We have a synthesis reaction, two compounds are linked to form one.
To balance the equation we must start by counting the atoms of each element on both sides of the reaction.
We have 2 atoms of Br on the reactants and 1 atom of Br on the products. We write coefficient 2 on KBr to have the same number of Br atoms.
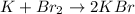
Now, we continue with the K atom. We write coefficient 2 on K molecule in order to have the same number of K atoms. We will have:
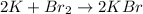
Now, the equation is balanced.
Answer: 2K + Br2 → 2KBr