Given:
Jill mixes two types of concentrations of HCI (hydrochloric acid):
a.) 0.375 liters of 25% hydrochloric acid and 0.625 liters of 65% hydrochloric acid.
To be able to find the final HCL concentration, we will be generating the following formula:
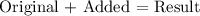
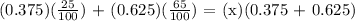
Where,
x = the final concentration of HCL
Let's find x,
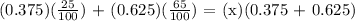
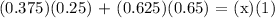
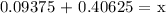

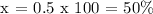
Therefore, the final concentration of the mixed solution is 50%.