To solve this question, we need to use the following formula:
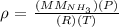
where:
d = density
MMNH3 = molar mass of NH3 = 17 g/mol
P = pressure = 800 Torr = 1.05263 atm (just divide by 760)
T = 25 °C = 273.15 + 25 = 298.15 K
R = 0.082 atm
So:
d = 17 * 1.05263/0.082 * 298.15
d = 17.8947/24.4483
d = 0.7319 g/L
Answer: density = 0.7319 g/L