To determine the moles of a compound from the mass in grams, we must use the molecular mass. The molecular mass is calculated by adding the atomic weights of the elements multiplied by the atoms of the element present in the molecule. To make it easier we can make the following table:
We have that the molar mass of CHCl3 is: 119.377g/mol.
The number of moles will be:
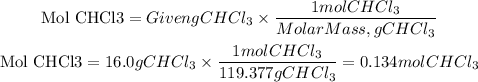
In 16.0g pf CHCl3 there are 0.134 moles