Answer:
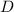
Step-by-step explanation:
Here, we want to get what happens to the phases of water as the temperature is decreased at 5 atm
From what we have in the question, we would be moving from left to right
What would happen here is that we move from the gaseous state to the liquid and then to the ice state
This means we have a change from a gas to a liquid then a solid