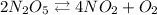We are given an equation that shows the differential rate change of the reaction and it is given as
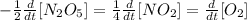
This depicts the decomposition reaction of N₂O₅. The forward reaction (product) carries a positive sign while the backward reaction or (reactant) carries a negative sign.
We can as well integrate this, but for the sake of simplicity, the equation of reaction is given as