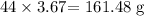Answer:
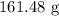
Step-by-step explanation:
Here, we want to get the mass of carbon (iv) oxide produced
From the question, we have the balanced chemical reaction stating that 2 moles of oxygen molecule produced 1 mole of carbon (iv) oxide molecule
The number of moles of carbon (iv) oxide produced from 7.34 mol oxygen is thus:
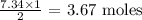
1 mole of carbon (iv) oxide contains 44 g
The mass in 3.67 moles will be: