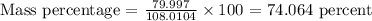So, To calculate the mass percentage of dinitrogen pentoxide (N2O5) we will first calculate the molar weight of the molecule. The mass percentage will be calculated with the following equation:
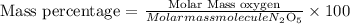
Molar mass will be:
Element Atomic Mass #Atoms Mass
N 14.0067 2 28.0134 g/mol
O 15.9994 5 79.997 g/mol
Total mass = 28.0134 + 79.997 = 108.0104 g/mol