ANSWER
The mass of hydrogen in 25g of (NH4)2S04 is 1.5152 grams
Step-by-step explanation
Given information
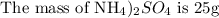
To find the grams of hydrogen, please follow the steps below
Step 1: Find the molar mass of (NH4)2SO4
Recall, that
The unit mass of nitrogen is 14u
The unit mass of hydrogen is 1u
The unit mass of sulfur is 32u
The unit mass of oxygen is 16u
![\begin{gathered} \text{ \lparen NH}_4)_2SO_4\text{ = \lbrack\lparen14 + \lparen1}*4)]*2\text{ + \lparen32\rparen + \lparen4 }*16) \\ \text{ \lparen NH}_4)_2SO_4\text{ = \lparen14 + 4\rparen}*2\text{ + 32 + 64} \\ \text{ \lparen NH}_4)_2SO_4\text{ = 18}*2\text{ + 32 +64} \\ (NH_4)_2SO_4\text{ = 36 +32 +64} \\ \text{ \lparen NH}_4)_2SO_4\text{ = 132 g/mol} \end{gathered}](https://img.qammunity.org/2023/formulas/chemistry/college/58tfc85a3kbkhb1uhndpsz8ls0luuhi1wo.png)
From the calculations, the molar mass of (NH4)2SO4 is 132 g/mol
Step 2: Find the number of moles of the compound
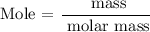
Mass = 25g
Molar mass = 132 g/mol
Substitute the given data into the formula above
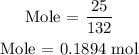
The number of moles of (NH4)2SO4 is 0.1894 mol
Step 3: Find the moles of hydrogen in the compound
There are 8 hydrogen atoms in (NH4)2SO4
Hence, we can find the number of moles of hydrogen below
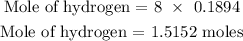
Step 4: Find the mass of hydrogen using the below formula
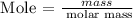
Recall, that the molar mass of hydrogen is 1.000 g/mol
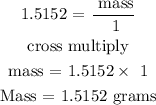
Hence, the mass of hydrogen in 25g of (NH4)2S04 is 1.5152 grams