1) List the compounds involved in the reaction.
Lead (II) nitrate
Potassium sulfate
Lead (II) sulfate
Potassium nitrate.
2) Write the formula of every compound.
Lead (II) nitrate: Pg(NO3)2
Potassium sulfate: K2SO4
Lead (II) sulfate: PbSO4
Potassium nitrate: KNO3
3) Write the chemical equation
.
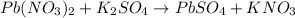
List the elements (or polyatomic ions) in the reactants.
Pb: 1
NO3: 2
K: 2
SO4: 1
List the elements (or polyatomic ions) in the products.
Pb: 1
NO3: 1
K: 1
SO4: 1
Balance K.
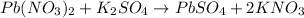
List the elements (or polyatomic ions) in the reactants.
Pb: 1
NO3: 2
K: 2
SO4: 1
List the elements (or polyatomic ions) in the products.
Pb: 1
NO3: 2
K: 2
SO4: 1
4) Balanced chemical equation.
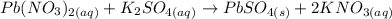
.