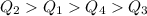Firstly, we need to remember how to find the liquid charge for a single atom. We can start by saying that
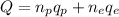
Where Q is the total charge, np is the number of protons, qp is the charge of a proton, ne is the number of electrons and qe is the charge of an electron
As we know, the charge of an electron is the opposite of the proton's. If we consider it as -1 and 1, respectively, we're left with
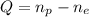
Then, let us find the charge for each atom.
So, for our atoms, we have
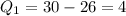
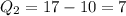
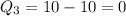
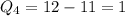
Then, our answer is