Final answer:
When 4.5 moles of O2 react, 9 moles of H2O are formed.
Step-by-step explanation:
The balanced reaction equation for the formation of water from oxygen is:
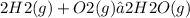
From the balanced equation, we can see that 2 moles of water are formed for every mole of oxygen consumed.
To find the number of moles of water formed when 4.5 moles of O2 react, we can use the mole ratio given by the balanced equation. The mole ratio is 2 moles of H2O : 1 mole of O2.
Therefore, we can calculate the number of moles of H2O formed as:
(4.5 moles O2) × (2 moles H2O / 1 mole O2) = 9 moles H2O