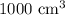
Step-by-step explanation
The volume of gas v held at a constant temperature in a closed container varies inversely with it's pressure P,in math terms it is
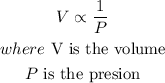
When Pressure is constant, Volume is directly proportional to temperature.
Step 1
given the data, we need to apply the formula for a constant temperature ,
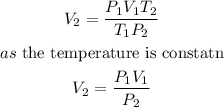
so
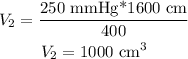
so, the answer is
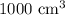
I hope this helps you