Firstly we would determine the oxidation number of the elements to determine which is undergoing oxidation and which is undergoing reduction:
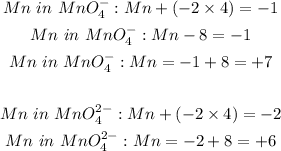
Mn oxidation number goes from +7 to +6 which means it is undergoing a reduction. A decrease in oxidation number means reduction.
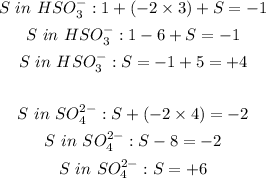
S oxidation goes from +4 to +6 meaning it is undergoing oxidation. There is an increase in oxidation number.
We will now balance the oxidation half reaction:
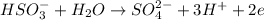
The coefficient for the water molecule is 1 in the balnced half reaction.