Step-by-step explanation:
The energy of a photon of the violet light can be obtained as follows:
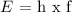
E = energy
h = Plank's constant
f = frequency
-------
Data provided:
f = 7.31x10^14 Hz
--
Data needed:
h = 6.63x10^-34 Js
So, E = h x f = 6.63x10^-34 Js x 7.31x10^14 Hz = 4.85x10^-19 J
Answer: 4.84x10^-19 J (nearest value)