In order to solve the question b):
We have the following reaction occuring:
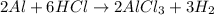
We need to calculate the number of moles of aluminum needed to produce 30ml of H2 (hydrogen gas) at a temperature of 22°C and a pressure of 763mmHg.
To calculate the number of moles of hydrogen produced we use the ideal gas equation:
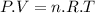
Where:
P is the pressure
V is the volume
n is the number of moles
R is the gas constant
T is the temperature
R is a constant and it's value is:
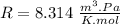
So we need to convert each variable to the units of this constant.
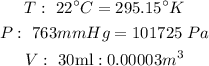
So now we calculate:
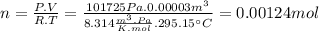
So we know that 0.00124 moles of H2 are formed.
Now we know that for every 2 moles of aluminum 3 moles of H2 are formed.
So we calculate the moles of Al needed:
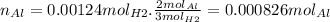
So the answer is 0.000826 moles of Al°