The equilibrium constant is given by the ratio between the products and the reactants, where their stoichiometric numbers are exponents.
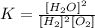
Observe that the molecule H2O must have a coefficient of 2 in order to balance the equation.
• [H2O] is the equilibrium concentration of the product (water).
,
• [H2} is the equilibrium concentration of the hydrogen molecule.
,
• [O2] is the equilibrium concentration of the oxygen molecule.