1.28x10^23 molecules of carbon dioxide are expelled.
1st) It is necessary to write the balanced equation of combustion from sugar:
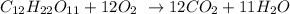
2nd) We need to look for the molar mass of C12H22O11 and CO2 to make a relation between those values:
- C12H22O11 molar mass: 342 g/mol
- CO2 molar mass: 44 g/mol
3rd) According to the balanced chemical equation, 1 mol of C12H22O11 produces 12 moles of CO2. Using the molar mass of the compound, in grams, 342g of C12H22O11 produces 528 g (44gx12) of CO2.
Now, with a mathematical Rule of Three we can find the amount of CO2 that is produced from 6.06g of C12H22O11:
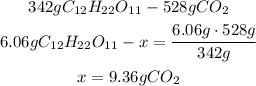
4th) Knowing that 6.06g of jolly rancher produces 9.36g of carbon dioxide, and using the Avogadro's number (6.022x10^23 molecules/mol), we can find the molecules of CO2:
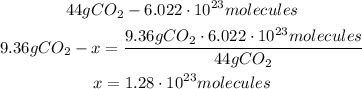
So, 1.28x10^23 molecules of carbon dioxide are expelled from the combustion of one jolly rancher.
We can write it in just one equation like this:
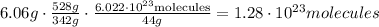
And the result will be the same.