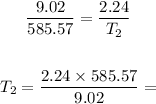Step 1 - Understanding the relation between volume and temperature for a gas sample
There are three main variables that can change the state of a gas sample: temperature, pressure and volume. If the pressure (P) is kept constant, the volume (V) becomes proportional to the temperature (T) in K:
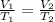
Step 2 - Substituting the values in the equation above
We know, from the exercise, that V1 = 9.02 L and T1 = 585.57 K, and V2=2.24 L. Substituting these values in the equation above: