Let's list down the given information.
Time = 75 hours
Final Value = reduced by 5% = 95%
To get the half life, the formula is:
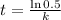
Before we can get the half-life, we need to get the value of k or the decay rate first. The formula is:
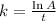
where A is the final value in percentage and t = time. Since we have this information above, let's plug it in the formula and solve for k.
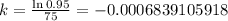
Now that we have the value of "k", let's solve for "t" using the formula stated above as well.
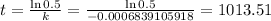
Hence, the half life of the radioactive substance is approximately 1,013.51 hours or 1,013 hours and 30 minutes.