Answer:
7910000 J
Step-by-step explanation:
The heat transfer to phase change is calculated as:
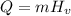
Where m is the mass and Hv is the heat of vaporization.
For water, Hv = 2260 J/g.
So, replacing m = 3500 g and Hv = 2260 J/g, we get:
Q = 3500 g (2260 J/g)
Q = 7910000 J
Therefore, the answer is:
7910000 J